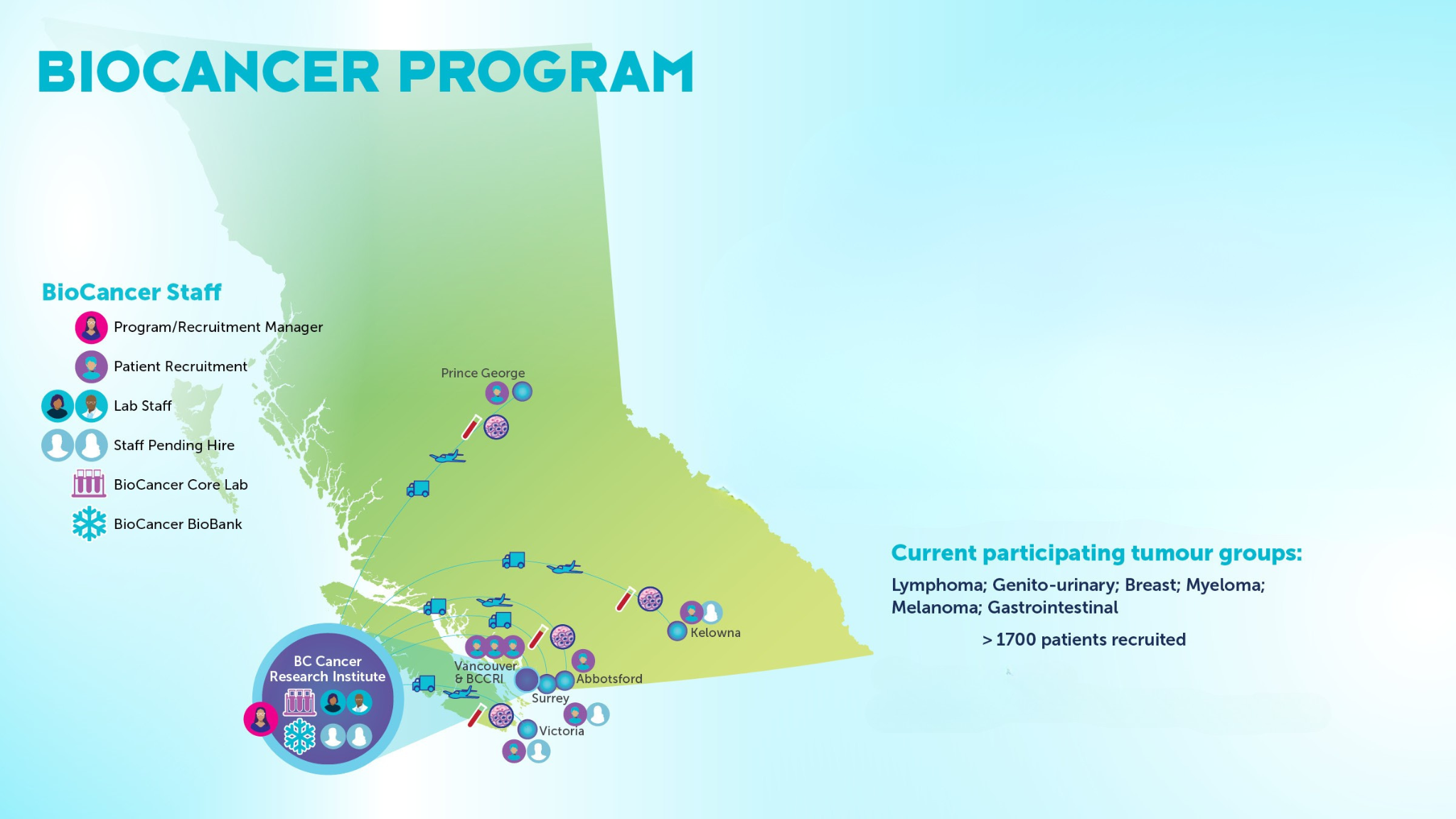
The BioCancer Program is a harmonized provincial biospecimen consenting, processing, storage and distribution platform for researchers across British Columbia. A collaboration between clinical and translational researchers, the program aims to advance scientific knowledge and enhance patient outcomes by improving detection, prevention and treatment of cancers in B.C. The BioCancer Program is administered by the Department of Clinical Research at the BC Cancer Research Institute.
Co-Leads
Dr. Stephen Chia
Department Head, Clinical Research
Dr. Christian Steidl
Executive Director, Research
Why is this important?
This new program provides a feasible operational infrastructure to increase equitable access to resources supporting biospecimen research, furthering cutting-edge research in B.C. The collection and use of biological samples – such as blood, urine, cerebrospinal fluid and tissue is essential for advancing cancer research. Through harmonized training and best practices for research staff, the program will promote quality across BioCancer research, and streamlined processes will increase efficiency and minimize duplication of effort between research groups.
What do we hope to achieve?
We hope to enhance population-based translational research by establishing a seamless, efficient and ethical infrastructure to acquire high-quality biospecimens obtained from as many eligible BC Cancer patients. Through the provision of biospecimens and acceleration of novel biomarker discovery and effective therapeutic development, we aim to improve patient outcomes in B.C.
Why participate?

The program will enhance the feasibility of research through services including consenting, centralized simple lab processing and short-term storage in a central freezer facility. This will support a fair and equitable process for researchers to participate in both defined prospective translational research and future biobanking research. This also includes a unified, integrated specimen cataloguing and tracking system for researchers to find or identify critical specimens, enabling the efficient use and re-use of samples and advancing the collaboration between researchers. Utilizing research assistants to support consenting and study-related tasks expands clinicians’ reach, increases enrollment capacity, and enhances the overall patient experience.
How will it work?

The Clinical Acquisition and Sample Administration research lab (CASA) is centrally located in the BC Cancer Research Institute and available for processing of various biospecimens using international Society for Biological and Environmental Repositories (ISBER) best practices. This includes the creation of standard operating procedures for all lab research staff including training and continued educational opportunities. Research assistants have been hired at all six BC Cancer regional sites to assist with screening, patient consenting and lab coordination tasks. All personnel will complete foundational and ongoing developmental training. This ensures proficiency in ethical consenting standards and study nuances.
Interested?
If you are interested in utilizing our services, please complete the BioCancer Tumour Group Intake Form or contact:
Gurpal Athwal, BioCancer Program Manager.