Fees, Timelines, Quality & Clinical Data
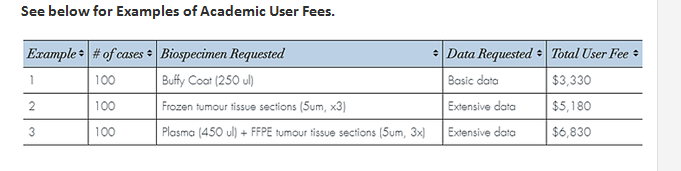
User Fees
The user fees for accessing the inventory are calculated based on the costs of biobanking modified by several factors including the number of cases and amount of biospecimen and extent of data requested. Discounts are applied to these costs for researchers supported by academic funding.
To see how costs are calculated visit the user fee calculator.
An estimate can be provided based on your completed application request. User fees for services including performing prospective custom collections are determined on a per study basis.
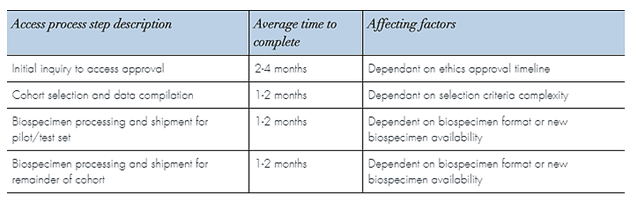
Access Timelines
Timelines are influenced by the level of biospecimen or data complexity within each study supported.
Factors include how promptly each step of the application process is completed and the complexity of the cohort or study selection criteria.
A time estimate can be provided based on your completed application Particular complexities exist with certain biospecimen formats that may require extra time to access and process. The complexity of the data fields requested may require extensive chart review and this can also extend the time to complete.
BBRS Menu
Overview
Researchers
Public
BC Cancer Foundation is the fundraising partner of BC Cancer, which includes BC Cancer Research. Together with our donors, we are changing cancer outcomes for British Columbians by funding innovative research and personalized treatment and care.